The third workshop on the roadmap to phase out animal testing for chemical safety assessments was organised by the European Commission (EC) and the European Chemicals Agency (ECHA) in Helsinki and via videoconference on June 16 and 17, 2025.
We offer a summary here.
All information, videos and presentations are available on the event page.
Why this worshop?
This workshop is the third of its kind, following those held in December 2023 and October 2024. Compared with the two previous ones, it provides further details on the timetable and governance.
This work was initiated in response to the European Citizens’ Initiative (ECI) “Save cruelty-free cosmetics – Commit to a Europe without animal testing” submitted to the European Commission in January 2023 (1.2 million signatories).
This roadmap should “show the way to accelerate the development, validation and implementation of non-animal methods”.
Three working groups have been set up:
- Human health
- Environmental safety
- Change management
In addition to the numerous meetings of these groups, 40 events (conferences, seminars, etc.) were held. And 2 online stakeholder surveys were conducted.
What does this roadmap cover?
The roadmap is expected to be around fifteen pages long, with a full document attached. It will cover all European legislation that may involve animal safety testing, i.e. 15 different pieces of legislation (Reach, biocides, pesticides, medicines, medical devices, veterinary products, products in contact with food, detergents, etc.).
Recommendations will cover the following points: replacing, reducing and refinement animal testing in the short and medium term; stages of implementation; facilitating the incorporation of new methods into legislation; setting up an effective validation/qualification network for new tests; choosing indicators to monitor implementation progress; choosing appropriate organizational structures.
Note the appearance of the new concept of “qualification”, which is similar to the concept of “validation”, but with a shorter procedure (without the inter-laboratory reproducibility verification stage). However, with this simplified procedure, products could be refused entry into certain countries for lack of international recognition of non-animal tests.
What are the deadlines?
The provisional timetable is as follows:
- Between June and September 2025, the roadmap project is drawn up, with discussions with stakeholders.
- Between September and December, the Commission will give its internal approval and adopt the roadmap, to be published in the first quarter of 2026, after which the implementation phase will begin.
- During the implementation phase, discussions will take place on the new technologies to be developed for the transition.
The heart of the work
The model proposed is that of the “three baskets”, specifying what is possible in the short, medium and long term:
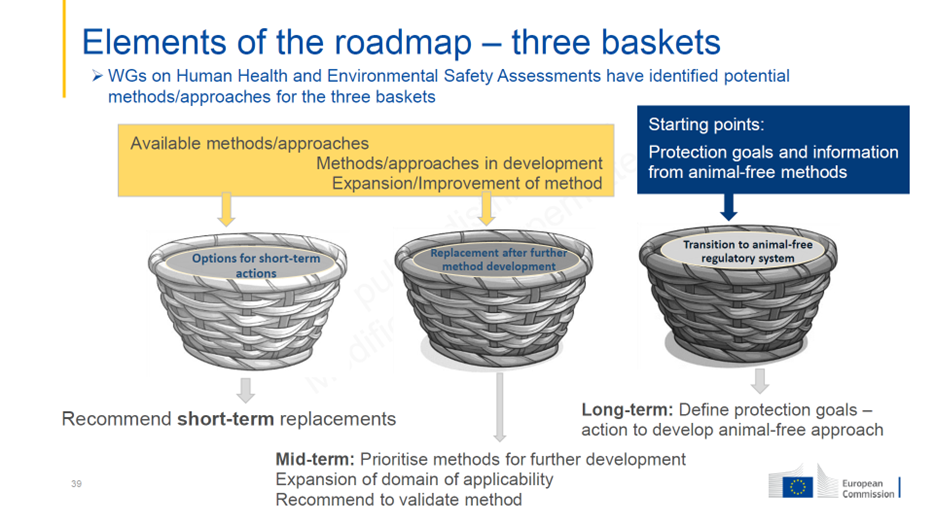
This approach is a useful way out of the sterile debate between those who want to replace all animal tests with NAMs (new methodological approaches) straight away, and those who argue that we’ll never get there. It’s a question of distinguishing between methods that can be used in the short term and those yet to be developed, in the medium or long term, in the complex fields of toxicology, bearing in mind that “one-for-one” replacement is not possible for most tests. Another approach is needed, summarized as “Next Generation Risk Assessment” (NGRA), based on a battery of in vitro and in silico tests.
Governance
Several bodies will be set up, with NGOs playing a full part, along with manufacturers, the EC and European agencies, and experts in non-animal methods. An important aspect of this governance is collaboration between all the European agencies involved.
Given the number of organizations and partners involved in this process, it is essential to coordinate the work so as to take account of other initiatives such as OSOA: “one substance, one assessment”, and the project to revise the Reach regulation on chemicals.
The roadmap should specify how all the organizations and bodies are to be coordinated. This role could be played by the European Partnership for Alternative Approaches to Animal Testing (EPAA).
Success indicators for the roadmap and its implementation will be implemented in a transparent manner:
- Indicators of commitment, knowledge sharing and trust-building
- Indicators on test development and validation, financing, cost-effectiveness, industry adoption, regulatory acceptance
- Indicators of barriers to adoption, efficiency and reliability
- 3Rs indicators
Problems and expectations of NAMs
During the first online stakeholder survey, at the end of 2024, 95% of nearly 200 respondents (manufacturers, NGOs, research institutes, regulatory authorities and agencies, etc.) saw benefits in developing the transition to animal-free testing: shorter time to market, lower cost, better corporate image, safer products, reduced administrative burden, etc.
But scientific obstacles remain, as does the barrier of regulatory acceptance.
That said, the obstacles to transition are not only technical, but also socio-cultural. It is important to create confidence in NAMs and to develop mutual trust between industry, scientists, administrative bodies and regulatory agencies.
The aim is to achieve a human-centered approach, without animal testing, adopting a mechanistic understanding of toxicity (causal links between exposure, molecular alterations, adverse effects), integrating environmental safety needs.
Short-term prospects include the idea of removing the obligation to test certain products on a “second species”, with a view to avoiding tests on dogs in particular.
The issue of funding for validation was identified as a key bottleneck. Several avenues were mentioned, such as seeking public/private partnerships (see the example of the PEPPER platform). It was also suggested that a sum dedicated to the validation of non-animal tests should be included in the amount allocated in European calls for projects.
Stakeholders’ expectations and priorities vary widely. For NGOs, the important thing is to stop using animals as quickly as possible. For scientists, in addition to this objective, the focus is on improving safety testing for human and environmental health. In view of the thousands of products to be tested in the future to meet safety requirements, it will be impossible to continue with animal testing in any case. We need to change our methodology, understand the toxicity process (which is different in mice and humans) and adopt a deterministic approach.
The example of the transition already made in the cosmetics industry is cited for inspiration. The EU could be a driving force in this area of alternative testing, but the USA is already well advanced. The need to make the transition at an international level was underlined.




