General framework
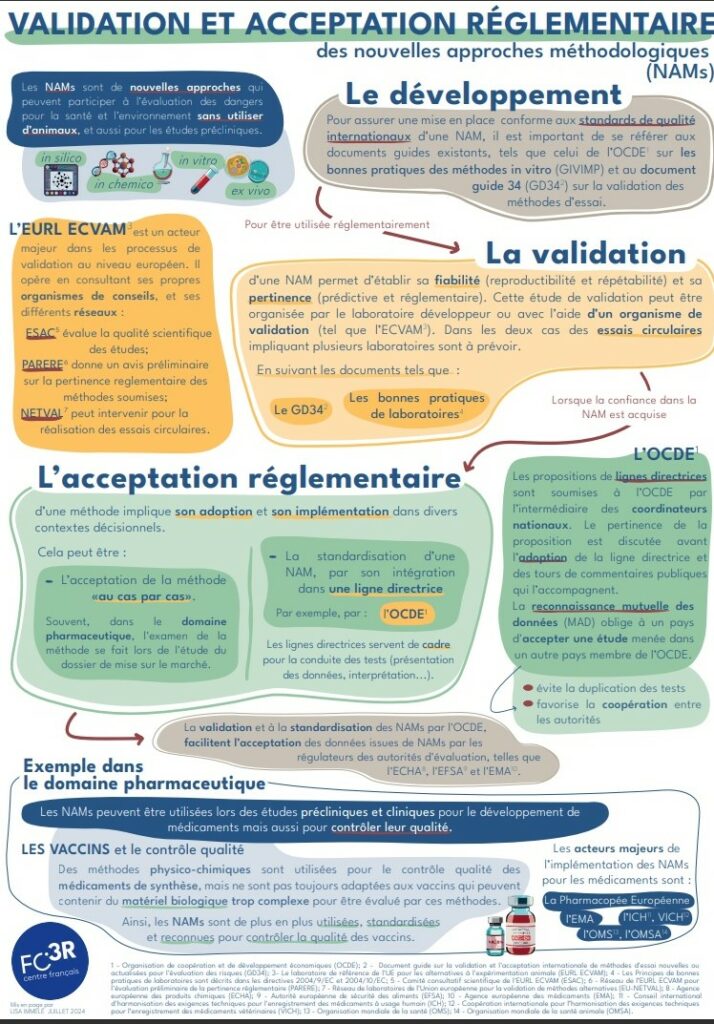
For alternative methods to be used in place of animal testing, they must first have been developed, recognized by the scientific community and validated.
In basic and applied research, substitute methods can be used freely, without any standardization constraints. These methods are scientifically recognized through the classic process of peer validation, i.e. publication in scientific journals. They are research tools and even objects of study. Using the term “replacement” is partly inappropriate, as they make an original and specific contribution to projects, without seeking to replace old methods using animals, which may eventually become obsolete.
These scientifically “recognized” methods can be used by manufacturers upstream of a regulatory validation process for screening, for example, avoiding animal testing and saving development time by eliminating highly toxic or ineffective molecules.
But when it comes to bringing a new product to market, a number of regulatory steps must be followed, in particular to guarantee the product’s safety for human health. In this context, alternatives to animal testing must undergo a validation process in order to standardize them and include them in the list of authorized protocols.
This international recognition is provided by the Organisation for Economic Co-operation and Development (OECD), in the form of guidelines, and/or the European Centre for the Validation of Alternative Methods (ECVAM) at European level, for chemical substances; the European Pharmacopoeia for drug quality control methods; and CEN/ISO standardization for medical media and devices.
This validation provides a guarantee of quality, enforceability and multilateral recognition.
When a research team wishes to have a regulatory test validated without animals, the process proves to be restrictive, long and costly, with no guarantee of ultimately obtaining validation of the test. Indeed, for an alternative method to be validated within the regulatory framework, it must provide, in addition to what is required for scientific recognition (proof of its relevance, its ability to detect the effects observed on humans and animals), proof that it can be reliably and reproducibly applied outside research laboratories.
The validation and regulatory acceptance circuit
Infographic produced by FC3R : download here




